Effect of LED lighting on growth and development of Atlantic salmon
Salmonids and many marine species react to light which plays an important role in their growth and development (Ref 1). Among others, Atlantic salmon is very sensitive to light, both for smoltification during the freshwater stage and during the on-growing stage in sea water. Using light to suppress early maturation during the on-growing stage has become a standard in the salmon industry (Ref 2). Yet, light impact on post-smolt salmon does not limit to only suppressing sexual maturation. Research studies show the influence of light on growth and development of salmon. Atlantic salmon post-smolts in sea water exposed to artificial light which provoked abrupt changes from natural short photoperiod to additional continuous light in the winter and spring period show significantly better growth due to the alteration of the seasonal growth cycle without triggering sexual maturation (Ref 3, 4, 5). However to date, lighting regimes in the salmon industry are far from being optimized in order to boost growth while preventing sexual maturation. Growth, development and onset of maturation appear to be connected, but the underlying morphological and physiological mechanisms are not completely clear yet. Although there are many signs that lighting can further support the growth, both fundamental research and the translation to commercial scale are often lacking. Philips supports both the fundamental research as well as bringing this knowledge applicable to farming practices.
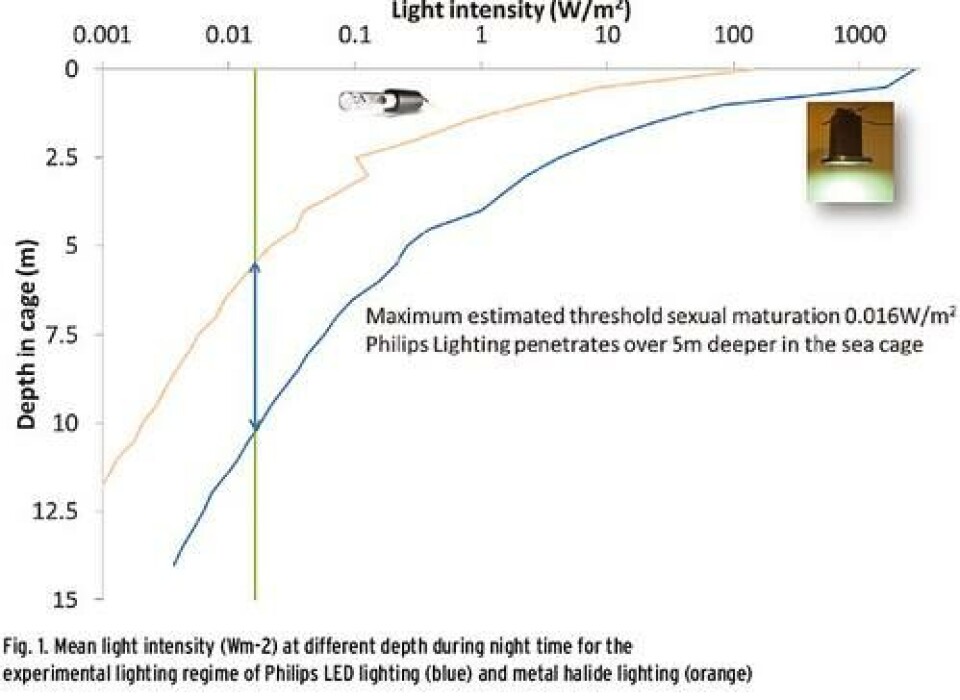
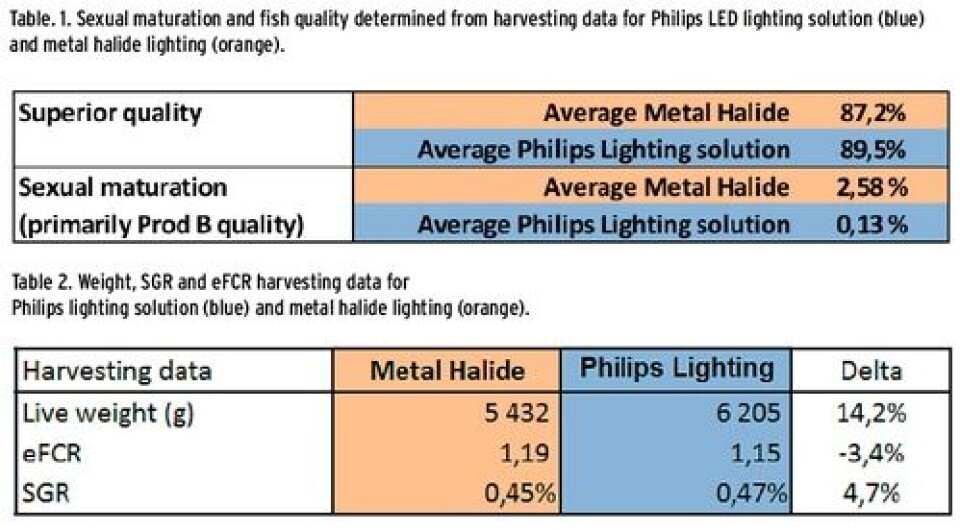
New possibilities of boosting growth and development while controlling sexual maturation are enabled recently by the use of specific LED lighting solutions during on-growing stage of Atlantic salmon. New lighting strategies beneficial for salmon farming are tested and implemented at commercial scales. The LED lighting used is specifically designed for this purpose: optimizing the spectrum of the light source, the intensity and light distribution throughout a cage (Fig.1). In order to reduce stress and improve light accommodation, dimming of light is implemented in the solution. The results of these trials are very promising and generate increased awareness and interest from the industry. This article presents the results of a trial conducted by Leroy and Philips at Gildeskål Research Station. The trial has been designed and organized by Leroy with the newly designed LED lighting system from Philips. The trial has been conducted according to industry standards in 90 m circumference cages. The trial started in December 2012 with 2 cages accommodated with LED lighting and 2 cages accommodated with metal halide lighting as control group. To maintain stocking densities at appropriate levels for optimum growth, the fish in each cage was split in May 2013, keeping the fish from LED cages and metal halide cages separated. The lights were turned off and the fish deloused prior to the splitting. At the start of the trial, in December 2012, the fish weighted 1.1 kg. During the trial fish was fed close to satiation using standard commercial diet. Along the trial the weight of the fish was continuously measured using AquaMetric weight-frames. The result of the trial has been evaluated based on harvesting data, continuous measurements of the fish weight and monitoring of the feeding and mortalities. Based on harvesting data, the trial shows that sexual maturation is significantly lower for the fish in the LED cages compared with metal halide cages (Table 1).The lower level of sexual maturation of the fish in LED cages has increased the amount of superior quality of compared to the metal halide cages, which translates into increased profit for the farmer. Over the period of winter and spring the fish kept in the LED lit cages had a significant lower mortality compared to the fish in the cages with metal halide. According to Johan Johansen, R&D manager of Gildeskål Research Station “We have used lights during winter and so far assumed that the proportion matured fish is unavoidable background noise, but the solution from Philips close to eliminated all maturation. With good salmon prices the return on investment is very short”
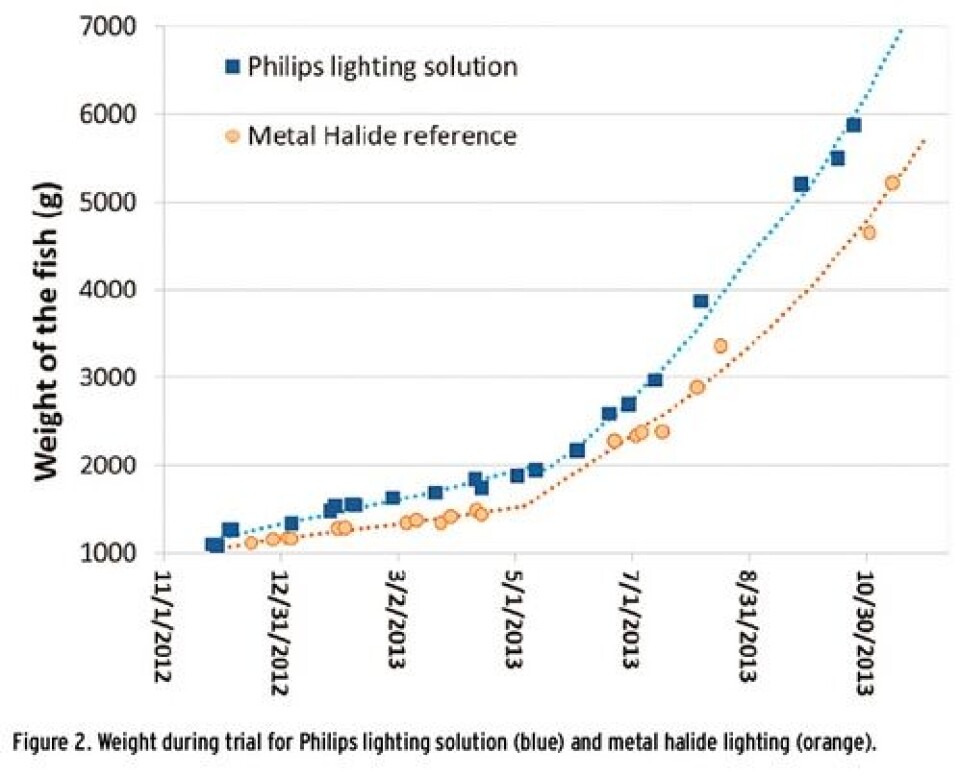
Both the salmon development expressed in weight and SGR and the economical FCR have been compared over the trial period. Evaluation has been done based on data collected along the trial, e.g. feed, fish weight, temperature and harvesting data which is most used in the industry. The results overview is presented in Table 2. According to analysis of the data, the mean weigh is significantly higher with ~14% in the LED group compared to the metal halide group (Figure 1), while the growth rate is higher with 4.7% in the LED group. Economical FCR shows 3.4% better results for the LED lighting based on harvesting data. This has a significant impact on reducing salmon production costs. According to Prof. Herve Migaud, Director of Research of the Institute of Aquaculture at the University of Stirling, “these results clearly showed an increased biological efficiency including suppression of maturation and enhancement of growth as compared to metal halogen. The new LED systems performed even better than expected. The use of these new systems commercially could contribute to boost productivity while improving fish welfare at sea. This is a very promising development that now requires commercial scale validation.”

And this is not the end of the journey of improving lighting regimes for the benefit of Salmon farming. Recently the impact of light is further investigated in other applications such as environmental manipulation of salmon swimming depth in order to reduce sea lice infection in Atlantic salmon farms (Ref. 6), biomass density control (Ref. 7), fish brain development (Ref. 8).
This article shows how fundamental research can be translated into tangible solutions to be applied by farmers and bridging the gap of fundamental research and the farming operations. The lighting equipment needed is now commercially available to be applied. New developments show that with this success also new research can be funded to fuel a next wave of innovative solutions. The final goal is to create a healthy and sustainable ecosystem of knowledge institutes, suppliers and farmers for the benefit of society such as lowering the environmental footprint of the industry, improve fish welfare and production practices, and become more efficient and cost effective.
References 1. H. Migaud, J.F. Taylor, G.L. Taranger, A. Davie, J.M. Cerdá-Reverter, M. Carrillo, T. Hansen, N.R. Bromage. A comparative ex vivo and in vivo study of day and night perception in teleosts species using the melatonin rhythm, J. pineal res. 41 (1), 42-52, 2006. 2. G. L. Taranger, M. Carrillo, R. W. Schulz, P. Fontaine, S. Zanuy, A. Felip, F.-A. Weltzien, S. Dufour, Ø. Karlsen, B. Norberg. Control of puberty in farmed fish, Gen. Comp. Endocrin. 165 (3), 483-515, 2010. 3. F. Oppedal, G. L. Taranger, J. Juell, J. E. Fosseidengen, T. Hansen. Light intensity affects growth and sexual maturation of Atlantic salmon Salmo salar L. postsmolts in sea cages, Aquat. Living Resour. 10, 351–357, 1997. 4. H. P. Endal, G. L. Taranger, S. O. Stefansson, T. Hansen. Effects of continuous additional light on growth and sexual maturity in Atlantic salmon, Salmo salar, reared in sea cages, Aquaculture 191, Issue 4, 337–349, 2000. 5. U. Nordgarden, F. Oppedal, G. L. Taranger, G.-I Hemre, T. Hansen. Seasonally changing metabolism in Atlantic salmon (Salmo salar L.): I. Growth and feed conversion ratio, Aquaculture Nutr. 9, 287–293, 2003. 6. B. Frenzl, L.H. Stien, D. Cockerill, F. Oppedal, R.H. Richards, A.P. Shinn, J.E. Brona, H.Migaud. Manipulation of farmed Atlantic salmon swimming behaviour through the adjustment of lighting and feeding regimes as a tool for salmon lice control, Aquaculture 424–425, 183–188, 2014. 7. F. Oppedal, T. Dempster, L. H. Stien. Environmental drivers of Atlantic salmon behaviour in sea-cages: A review, Aquaculture 311 (1), 1-18, 2011. 8. L. O. E. Ebbesson, V. A. Braithwaite. Environmental impacts on fish neural plasticity and cognition, Journal of Fish Biology 81, 2151-2174, 2012.