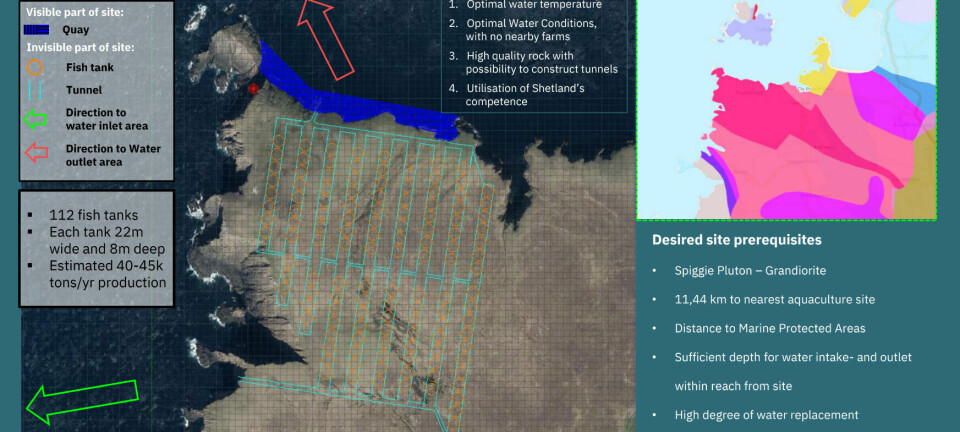
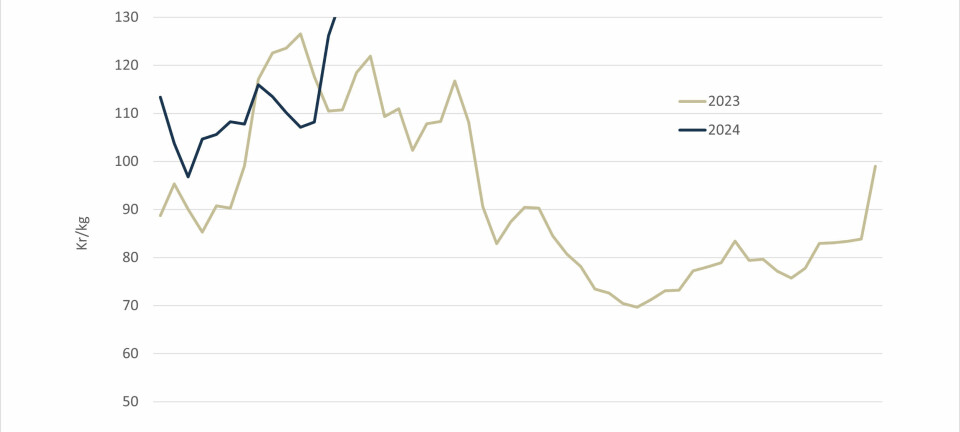
Survey shows support for controversial treatment
More than three-quarters of salmon producers in Scotland say medicinal feed premix Slice is ‘valuable’ to their business, according to a new survey by MSD Animal Health.
The survey follows this month’s announcement by the Scottish Environment Protection Agency (Sepa) that is to review the licences of all Scottish fish farms using Slice, which targets sea lice in salmon and has the active ingredient emamectin benzoate.
A statement from MSD Animal Health said the aquaculture team’s first industry survey was sent to a wide selection of customers and industry stakeholders in a bid to further develop insight and improve relationships.
“With results now in, the MSD Animal Health team has been able to gain sight of what value the products used are to a business, what issues are being discussed in the industry and where they can offer increased support and value to its customers,” the statement said.
MSD Animal Health's survey of its customers found more than 78 per cent believed Slice was valuable to their business, while 74 per cent rated Aquavac PD3, a single-injection vaccine that protects against the three main pathogens affecting salmon in the UK, as medium to high value.
Respondents in the survey also said that additional input would be welcome in terms of staff training, vaccination planning and biosecurity.
Positive feedback

Dafydd Morris, business manager at MSD Animal Health Aquaculture, said: “We produced this survey as part of our commitment to investing in research and development to improve fish health in farmed salmon. We strive to continually improve our services, so to gain insight like this from the survey is an excellent way to listen to and learn directly from our customers.
“We work with fish farms on a daily basis and with the results of this survey reflecting the positive feedback we receive face-to-face, it is very encouraging. It also provides us with insight where our services such as staff training can be further utilised and brought in to improve the services we offer.
“Our team will use the results and additional feedback to enhance the product development and services offered across the country in order to continually improve fish health.”
Crustaceans affected
Earlier this month, a Sepa spokesman told fishfarmingexpert.com: “A study by the Scottish Aquaculture Research Forum, completed last August, confirmed a subtle but detectable, and unexpected, association between impacts on the marine environment and the use of Slice, where very low concentrations of the medicine may have affected crustaceans in the seabed.
“Based on this new evidence, Sepa is reviewing all fish farm licences permitting the use of Slice, tightening conditions for the medicine’s use after discussions with the Veterinary Medicines Directorate.
“We are now beginning the issuing of these new licences and this will be completed by the end of April. This restriction will remain in place while Sepa and the industry carry out further research to either confirm or confound the apparent link between Slice use and possible environmental effects.”
“We are also now considering the findings of a review we commissioned of the environmental quality standards for Slice to ensure they are up to date and provide adequate environmental protection.”
MSD Animal Health is known as Merck Animal Health in the US and Canada.